Abstract
Background
Myelofibrosis (MF) is a clonal hemopoietic stem/progenitor cells (HSPCs) disorder with mutations in 3 driver genes (JAK2, MPL, or CALR) and inflammatory microenvironment. Triple negative (TN) patients (pts) do not carry the 3 driver mutations and display a significantly worse survival-rate which can be partially due to a greater mutation complexity. Circulating microvesicles (MVs; 0.1-1 μm), most of megakaryocytes (MKMVs) and platelets (PMVs) origin, play a role in intercellular signalling and are increased in inflammation and cancer including MF. We hypothesized that in TN pts the HSPCs and/or the inflammatory microenvironment show a more aggressive and malignant phenotype.
Aims
To identify a biomolecular signature of TN pts by comparatively evaluating the circulating HSPCs behaviour and their functional interplay with the inflammatory microenvironment.
Methods
EDTA-anticoagulated peripheral blood (PB) was collected from 16 MF pts (WHO-2016 criteria-JAK2(V617F) mutated (n=10) and TN (n=6)) and 20 age/sex-matched healthy donors (HD). Driver mutation status was obtained by PCR/NGS. Plasma MVs were isolated by ultracentrifugation (45.000 rpm for 2 hours), counted by Nanosight technology and phenotypically characterized for PMVs (CD61+CD62P+) and MKMVs (CD61+CD62P-) by flow cytometry (size 0.5-0.9 µm). in vitro survival (24 hours), CXCL12-driven migration (12 hours) and clonogenic ability of immunomagnetically isolated CD34+ cells from pts PB or cord blood (CB; n=10) was performed in the presence/absence of the isolated MVs or IL-1β, TNF-α and IL-6 (alone/ in combination). Incorporation of CFSE-labelled MVs into CD34+ cells was analyzed by confocal microscopy. MicroRNA (miRs) profile analysis of the isolated MVs and Gene expression profile (GEP) analysis of CD34+ cells from 3 JAK2(V617F) mutated, 3 TN pts and 3 HD/CB was performed. Plasma inflammatory cytokines levels were evaluated by ELISA.
Results
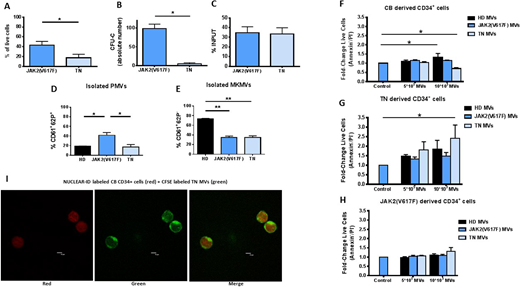
We firstly compared the TN and JAK2(V617F) CD34+ cells. TN CD34+ cells showed decreased in vitro survival and clonogenic ability but comparable migration capacity (Fig. 1 A, B, C). Consistently, comparing the GEP, the expression of selected anti-apoptotic (TSPYL5, GFI-1 and FCMR) and pro-apototic (TNFSF10, TP53INP1) genes was significantly down- and up-regulated, respectively, in TN CD34+ cells. We then investigated whether signals from the microenvironment may affect the in vitro behaviour of the TN CD34+ cells. IL-1β, IL-6 and TNF-α plasma levels were significantly increased (p<0.05) in MF pts. However, at variance with the JAK2(V617F) mutated counterparts, survival and clonogenic ability of TN CD34+ cells were not significantly promoted by IL-1β, IL-6 and TNF-α, alone or in combination. This was due, at least in part, to the reduced expression of IL-6 and TNF-α (type I) receptors on TN CD34+ cells. We then analyzed the phenotype of the isolated circulating MVs showing that the PMVs were significantly increased (p<0.05) in the JAK2(V617F) mutated pts only. Conversely, the MKMVs were significantly decreased in both pts groups (p<0.01) as compared to the HD counterparts (Fig. 1 D, E). When we co-cultured CD34+ cells with the isolated MVs we found that TN MVs significantly increased the survival of TN CD34+ cells but inhibited CB CD34+ cells survival (p<0.05, respectively). Conversely, JAK2(V617F) MVs did not significantly affected the survival of CB/pts CD34+ cells. HD MVs significantly increased the survival of CB CD34+ cells (p<0.05) only (Fig. 1 F, G, H). Of note, MVs from pts were transferred into cytosol after co-cultured with CB CD34+ cells (Fig. 1 I). Consistently, comparing the miRs profile of pts and HD MVs, various pro-apoptotic (+) and anti-apoptotic (-) miRs were upregulated. Specifically, miR-155(-), 24(-) and 222(-) were upregulated in TN MVs only; miR-21(-), 19a(-), 34a-5p(+), 423-5p(+) were upregulated in both JAK2(V617F) and TN MVs. Further, miRs regulating inflammation (miR-146a) and proliferation (miR-1274A, downregulated in TN vs JAK2(V617F) MVs), were overexpressed in pts MVs.
Conclusions
Our results demonstrate that TN CD34+ cells show in vitro defective function and are unresponsive to the inflammatory microenvironment. Interestingly, only the autologous circulating MVs promoted the malignant clone, suggesting that MVs, as signals from microenvironment, may have a pathogenetic role in TN MF and may be the target of novel therapeutic approaches.
Fabbri:Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS: Employment. Cavo:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Palandri:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal